
上場を果たした革新的創薬ベンチャーの新たな決意
人間の肝臓に備わった、驚異的な再生力。その鍵をにぎる「HGF(肝細胞増殖因子)」を用いた難治性疾患の治療薬に、大きな期待が寄せられている。
世界で唯一、HGFを医薬品グレードで量産する技術を持つ創薬バイオベンチャーとして、2020年末に東京証券取引所マザーズ市場への上場を果たしたクリングルファーマ。
KIIの投資先として初となる上場達成への道のりについて、代表取締役社長の安達喜一とKIIの投資担当者・本郷有克が振り返り、新たな決意に想いを馳せる。
肝臓を再生する「HGF」を用いた、世界で唯一の創薬技術
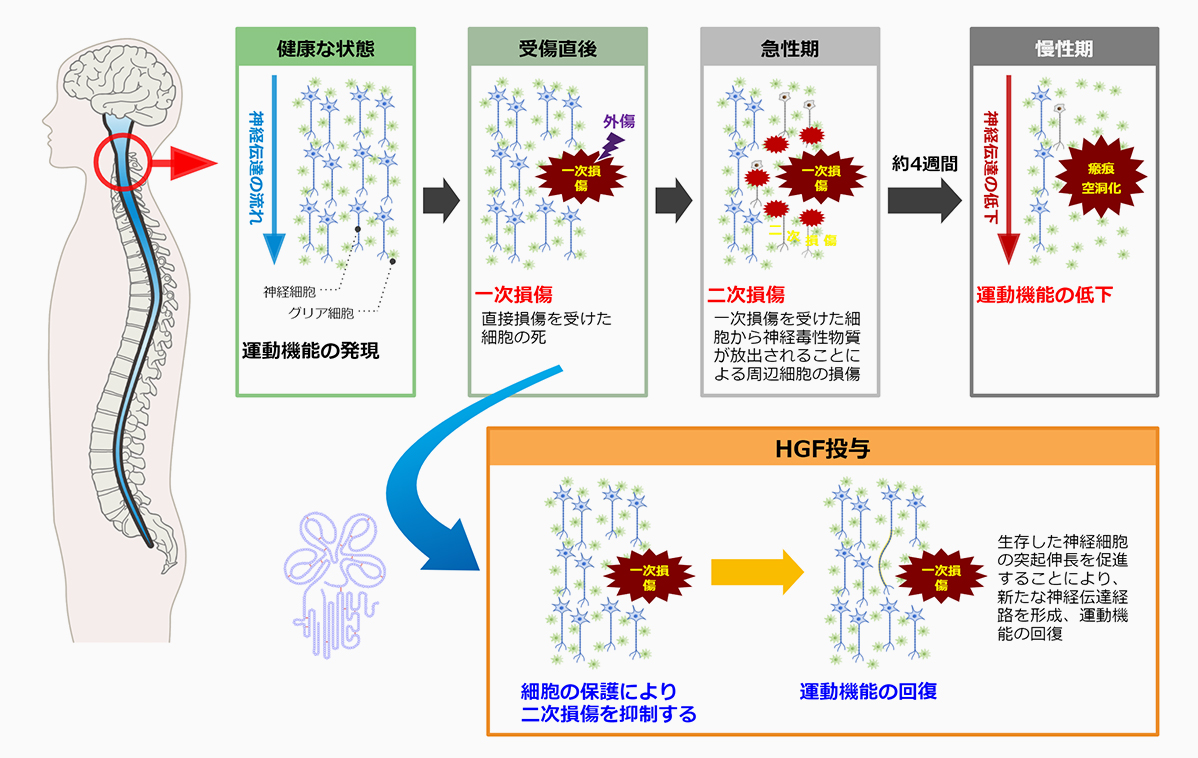
安達: 弊社は大阪大学発の創薬バイオベンチャーとして、人間の体内に存在する「HGF(肝細胞増殖因子)」というタンパク質を用いた医薬品開発に取り組んでいます。肝臓は人間の体の中で最も再生能力が高い臓器です。例えば生体肝移植の提供者の場合、肝臓の1/3を切除しても再生することが知られていますが、そのメカニズムは長らく解明されないままでした。HGFはこの肝臓の再生に関わるタンパク質として、1984年に大阪大学医学部の中村敏一先生によって発見され、その後の研究によって人体のさまざまな組織や臓器の再生・修復を担うことが明らかになり、中枢神経系にも強力に作用することがわかったのです。
弊社は世界で唯一、このHGFを医薬品グレードで量産する技術を実現しました。現在は数種類の難治性疾患に対し、HGFによる治療の臨床試験を実施しています。例えば、第1パイプラインの脊髄損傷急性期については治療薬として申請・承認前の最後の段階であるフェーズ3試験を、第2パイプラインの筋萎縮性側索硬化症(ALS)については患者さんに対して有効性を確認するフェーズ2試験を行っているところです。

本郷: 私が安達さんに初めてお会いしたのは、KIIの設立から数カ月後の2016年春のことでした。HGFを用いた脊髄損傷急性期の臨床試験に携わられている、慶應義塾大学医学部の中村雅也教授のご紹介でお話をうかがったのですが、ベンチャー企業でありながらこれだけ画期的な治療薬の知見や技術を培ってきたということに驚かされました。私自身、かつては再生医療の研究や製薬企業との研究開発業務に携わっていたこともあり、日本の製薬業界の環境を含め、ベンチャー企業が高いレベルで研究開発を維持し続けることの大変さを、折に触れて感じてきたからです。
安達: 実はその頃、弊社は非常に厳しい状況にありました。ちょうど脊髄損傷急性期についてフェーズ1/2の臨床試験を実施していたのですが、患者さんの組入れに予想外に時間がかかり、資金不足に陥っていたのです。弊社は2001年創業で創薬ベンチャーとしては社歴も長く、数多くのベンチャーキャピタルからすでに出資を得ており、新たな出資元が見つからない状態でした。社内では、資金調達ができなければ会社をたたむしかないという意見も出ていたところ、慶應義塾大学がベンチャーキャピタルを設立するという話を耳にして、脊髄損傷急性期プロジェクトを共同で進めていた岡野栄之教授と中村雅也教授にご紹介いただいたというわけです。
本郷: クリングルファーマはその時点で、すでに数多くのデータを積み上げて臨床研究に取り組んでいました。このまま進んでいけば良い結果が出ることを裏打ちするデータもありましたし、安達社長をはじめとする社員の方々のプロジェクトに懸ける想いも、投資を決定付けた大きな要因になったと思います。そしてこれが、KIIにおける私の初投資案件になりました。
安達: 私たちとしてはそれが、ギリギリの状態で手を差し伸べていただいた形になりました。その結果、無事に脊髄損傷急性期のフェーズ1/2試験を完結させ、患者さんに対する有効性を示唆するデータを得ることができたのです。投資契約は16年の12月のことで、同じ月に私も社長に就任するなど、私自身にとっても大きな転機になりました。
創薬ベンチャーとVC、新薬実現に向けた協働の日々
ーー投資後は、お互いにどのような体制でプロジェクトを進めていったのでしょう?
安達: 本郷さんにはいわゆるベンチャーキャピタリストのイメージを超えて、創薬関連の豊富な経験と知識を生かしながらハンズオンの支援をしていただきました。資金面での支援だけでなく、さまざまな企業との対外的な交渉はもちろん、海外においても英語での商談を取りまとめていただくなど、私たちにとって大きな力になっていただいたと感じています。
本郷: ありがとうございます。新しい薬を開発する上で、大きな製薬企業であれば研究開発から臨床試験、販売など各方面のエキスパートが所属しており、組織的に力を結集して取り組むことが可能ですが、ベンチャー企業はそうした人材を自社内でまかなうことができません。その状況に対して少しでもお手伝いできればと思い、なるべく時間をかけて支援に取り組んできました。

安達: 印象に残っているのは、ハーバード大学発のバイオベンチャーであるクラリス・バイオセラピューティクス社とのビジネス・ディスカッションの時のこと。サンフランシスコのホテルで先方の社長と契約書を見ながら細かい内容を詰めているなかで、私がキャッチアップしきれないところを本郷さんがその場で丁寧にアドバイスしてくれました。長い時間をかけての交渉になりましたが、こうした念入りなやりとりがあったからこそ、同社に対するHGFタンパク質のライセンスと原薬の供給契約を実現することができたのだと思います。
本郷: その時のことは私もよく覚えています。懐かしいですね。創薬関連の契約は内容が非常に多岐にわたるため、例えば法律に関する部分は弁護士に依頼するなど専門家の確認が必要ですが、私がわかる範囲でクリングルファーマが保持すべき権利をきちんと盛り込めるようにチェックをしたり、一緒に交渉に臨んだりしてきました。また私自身、社外取締役として取締役会議にも参加させていただき、IPOを目指すための組織体制についてなど、率直な意見をお話しする機会もありました。
安達: 弊社にとってIPOは重要なマイルストーンですが、私たちの目標はあくまで薬を世に出すこと。そのことを深く理解された上で的確な意見をいただいたことが、これまでの成果につながったと感じています。またIPOに向けた想いとしては、これまでプロジェクトを支えてくださった投資家の方々に株を売却できる機会をつくることが、私たちの果たすべき責任の一つだと肝に銘じてきました。そこへ至る道筋という意味でも、本郷さんのアドバイスには常々、大きな力をいただきましたね。
本郷: でも、私自身はあくまで支援をする立場にすぎません。IPOに向けて着実に成果を上げてこられたのは、安達社長の決断や与件達成の賜物だと思います。
上場達成を経て語られる、新たな使命と創薬の未来
ーーこうした努力の結果、20年12月28日に東京証券取引所マザーズ市場への上場を達成されました。どんな想いで上場を迎えられましたか?
安達: 新型コロナウイルス感染症の影響で開催中止の可能性もありましたが、上場の瞬間に鐘を鳴らすセレモニーに出席できたことは、感慨深い経験でした。とはいえ、喜びよりも「この気持ちを励みに、薬を世に出すところまでやり切らなければ」と、今後の責任がひときわ重く感じられたのを覚えています。というのも、ここから会社の成長をさらに加速させていくのが私の役割だからです。薬の実用化に向けて承認を得たり、海外での提携先を獲得したりと、これからは一般の株主の方々に対してもきちんと実績を上げていかなければならない。このことに尽きると思います。

本郷: KIIのベンチャーキャピタリストとしてのクリングルファーマとの関わりは、IPOを達成した時点で完了となりました。私が初めて担当した会社であり、KIIとしても投資先ベンチャーの初めての上場達成ということでいろいろな想いがありますし、私自身も5年間のやりとりを通して、多くの知識や経験を身に付ける機会に恵まれたと感じています。その想いを、これからは新たな投資先のIPOに向けて捧げていきたい。それが私たちの使命だと考えています。
安達: 16年当時から考えても、最短ともいえるスピードでIPOを達成できたのは、まさに本郷さんや多くのみなさまのご支援あってのことです。本当に、感謝の念が尽きません。何より本郷さんにはお会いした当初から、お互いに立場は違っていても、自分の仕事を通じて世の中に貢献したいという根本的なビジョンや想いを共有することができる方だと感じてきました。
本郷: それは私も同じです。リードVCとして既存の投資家と意思の疎通を図ったり、新規の投資家の方にはクリングルファーマに投資する意義をご理解いただくお手伝いをさせていただいたりと、安達社長をはじめ会社の方々と二人三脚で力を合わせることができた経験は、自分にとっても大きな財産になったと実感しています。
ーークリングルファーマのこれからのビジョンについて教えてください。
安達: 短期的には、第1パイプラインである脊髄損傷急性期のフェーズ3試験を終了させ、弊社として初めてとなる薬事承認を得ることです。これに続いて、ALSや声帯瘢痕、急性腎障害といった他の疾患についても臨床試験を進めていく。中期的には、難治性疾患に対する適用範囲を拡大しながら海外の製薬会社とも提携を広げるなど、HGFが持つ価値と可能性を最大化していこうと考えています。
そして長期的には、自社で医薬品の開発から販売までを担うことができるバイオ製薬企業を目指して、この会社を成長させていきたい。欧米の製薬企業には、ベンチャーからスタートして世界的規模へと成長した例が数多く見られますが、こうした会社に共通している特徴の一つとして、自社で製品を展開していることが挙げられます。これに対して、開発した新薬を製薬企業にライセンスアウトしてしまうことは、その製薬企業に将来の収益を渡してしまうことに他ならないと私自身は考えています。だからこそ、まだ治療法のない疾患に苦しむ患者さんに向けて、私たち自身の手でよりよい薬を作り出し、届けていきたい。今後のさらなる研究開発のためにも、その想いを忘れずに努力していきたいと考えています。
本郷: ベンチャーにとって過酷な環境といわれる日本の製薬業界にとって、大きな希望につながるお話だと思います。私個人としても、製薬企業として成長を遂げたクリングルファーマの姿が見られる日を、心から楽しみにしています。

【関連記事】
クリングルファーマ 代表取締役 安達喜一 インタビュー「世界唯一の創薬技術で描く未来への希望」(2017年)
https://www.keio-innovation.co.jp/dialogue/369/